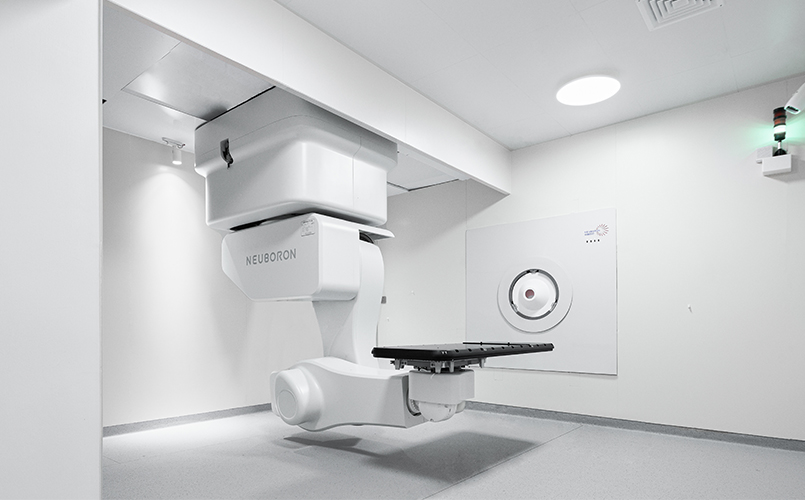
The historic moment has arrived. On April 23, Neuboron’s Phase I registration clinical trial of BNCT drug-device products was launched at Xiamen Humanity Hospital. This is the first BNCT registration clinical trial in China, marking a crucial step towards the registration, commercialization, and industrialization of China’s first BNCT system with independent intellectual property rights, the NeuPEX Block-I, and its domestic companion boron drug, NBB-001, putting China at the forefront of the world in this field.
The clinical trial is led by Professor Huang Cheng and Professor Pan Jianji from Xiamen Humanity Hospital, aiming to evaluate the safety and tolerability of BNCT using NBB-001 and the BNCT system in patients with recurrent head and neck malignancies. The devices and drugs used in this clinical trial are both made in China and provided by Neuboron, which also offers technical support. These include Neuboron’s self-developed medical device, the “Boron Neutron Capture Therapy System” (NeuPEX Block-I), and the trial drug “Borofalan [10B] for Injection” (NBB-001).

At the launch ceremony, Principal Investigator Professor Pan Jianji said, “BNCT is a cutting-edge tumor treatment technology that is being vigorously developed worldwide and must be mastered by ourselves. We hope that through the conduct of this clinical trial, we can form BNCT clinical research results with international influence and clinical data for Chinese patients, better guide domestic BNCT clinical practice, and contribute Chinese solutions and wisdom to the global development of BNCT.”
Prior to this registration clinical trial, the Xiamen BNCT Center has conducted preclinical irradiation experiments on multiple varieties and batches of animals, including mice, rats, and beagle dogs, as well as Investigator-Initiated Trials (IIT) in humans. So far, nearly 25 patients have been successfully treated, with indications including malignant brain tumors and recurrent head and neck malignancies. This has demonstrated the significant local control effect and safety of BNCT for gliomas and head and neck tumors, providing a solid foundation and rich experience for the launch of this registration clinical trial.
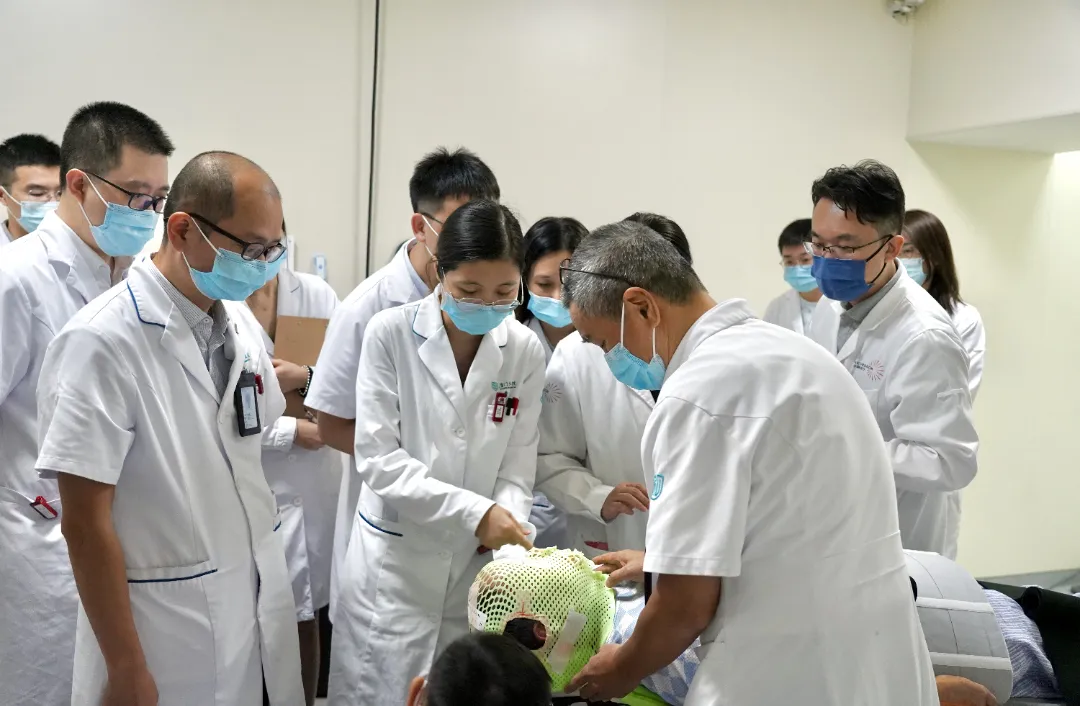
The launch ceremony coincides with the 30th National Cancer Prevention and Control Publicity Week. Moving forward, the medical and corporate parties will closely cooperate to accelerate the BNCT registration clinical process, enabling this cutting-edge tumor treatment technology to serve more patients as soon as possible. This will contribute to the construction of a cancer prevention and control system, eliminate the fear of cancer, and promote the realization of the grand vision of a Healthy China.