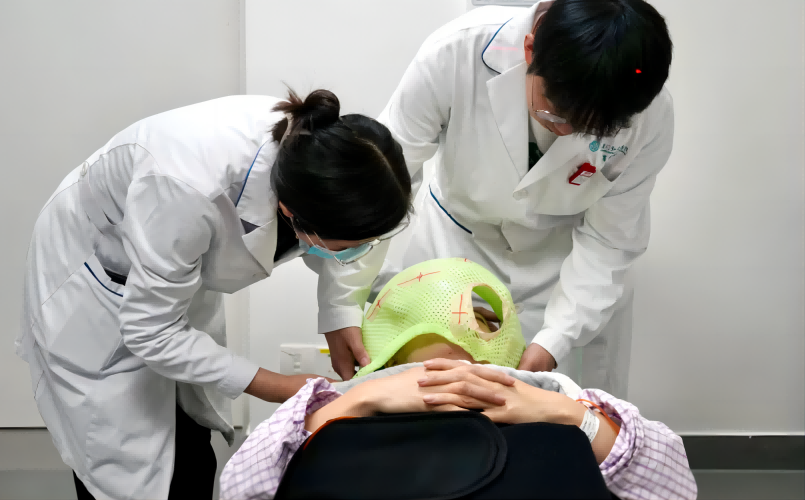
On May 22, 2024, Neuboron's BNCT drug-device innovative product completed the enrollment of the first patient in the Phase I clinical trial.
The first patient had previously undergone heavy ion therapy but unfortunately experienced tumor progression. The patient is hoping to regain health through the latest cutting-edge tumor treatment technology, BNCT.
Prior to BNCT treatment, the patient received a 2-hour intravenous infusion of boron drug, followed by neutron irradiation. During the treatment process, a team of doctors, nurses, physicists, and technicians worked together to ensure the patient's safety.
"I didn't feel any pain during the entire treatment process. I'm very fortunate to be the first to benefit from such advanced medical technology," the patient said sincerely after the treatment.
Seven days after BNCT treatment, the patient's condition remained stable under strict medical observation. Moving forward, the treatment team will follow up regularly in accordance with the clinical trial protocol.
Looking ahead, Neuboron will accelerate the registration and market launch of its domestic BNCT drug-device product, promote the clinical application of BNCT, and enable more head and neck cancer patients to benefit from this advanced tumor treatment technology.
Accelerator BNCT System Equipment
The BNCT equipment system (NeuPex) installed at Xiamen Humanity Hospital was independently developed by Neuboron and possesses complete intellectual property rights and patent technologies.
Under the conditions of a proton accelerator energy of 2.35 MeV and a beam current intensity of 10 mA, the superthermal neutron flux at the beam exit was measured to be 1.1×109n·cm-2·s-1 by the Beijing Institute for Medical Device Inspection, which is significantly better than the recommended standard value in the IAEA CRCP/BOR/002 report.
After passing the medical device registration inspection, the NeuPex system also obtained the formal medical device clinical filing approval from National Medical Products Administration (NMPA), and its product registration progress is leading in the country.
It is worth mentioning that the superthermal neutron flux per unit current of the NeuPex system can reach 1.1×108n·cm-2·s-1·mA-1, and the superthermal neutron flux per unit target proton beam power consumption is greater than 4.5×107n·cm-2·s-1·kW-1. This indicates that the system has a high efficiency of superthermal neutron flux conversion, meaning that the generated neutrons are more effectively applied in clinical treatment, and at the same time, the radiation impact on the site and system can be minimized to the greatest extent.
Trial Drug: Borofalan (10B) for Injection
The trial drug is " Borofalan (10B) for Injection" (NBB-001), an independently developed domestic boron drug by Neuboron. It is a broad-spectrum boron-containing drug transported via the L-amino acid transport system (LAT-1) and is intended for the treatment of recurrent malignant head and neck tumors. Non-clinical studies have shown that NBB-001, in combination with neutron irradiation, demonstrates a tumor inhibition rate of over 85%.