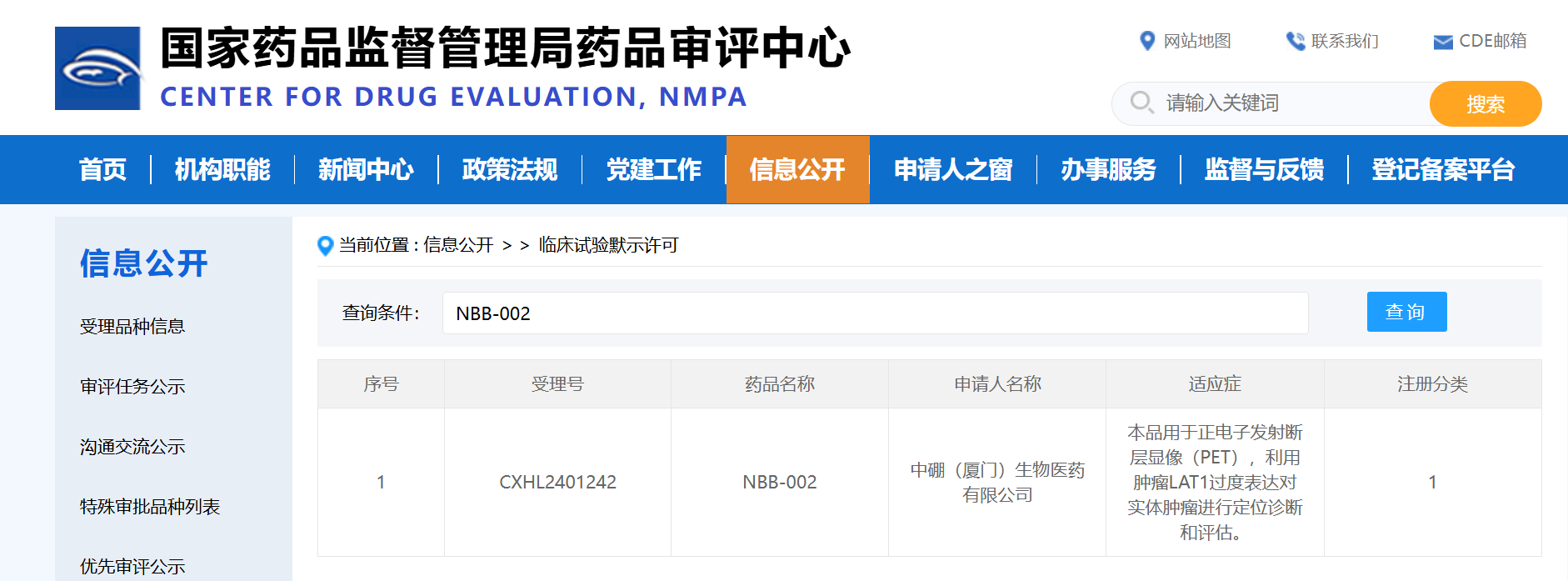
On January 26, 2025, the official website of the Center for Drug Evaluation (CDE) under the National Medical Products Administration (NMPA) announced that the clinical trial application for NBB-002, a Class 1 innovative drug independently developed by Neuboron, has been approved (Acceptance No.: CXHL2401242).
As a "global first" innovative drug, it is the world's first BNCT companion diagnostic drug approved to enter the registered clinical trial stage. It is also another major breakthrough achieved by Neuboron after the approval of the IND application for its self-developed BNCT therapeutic drug "Boron [¹⁰B] Melphalan for Injection" (BPA), becoming another landmark achievement in the field of BNCT clinical development.
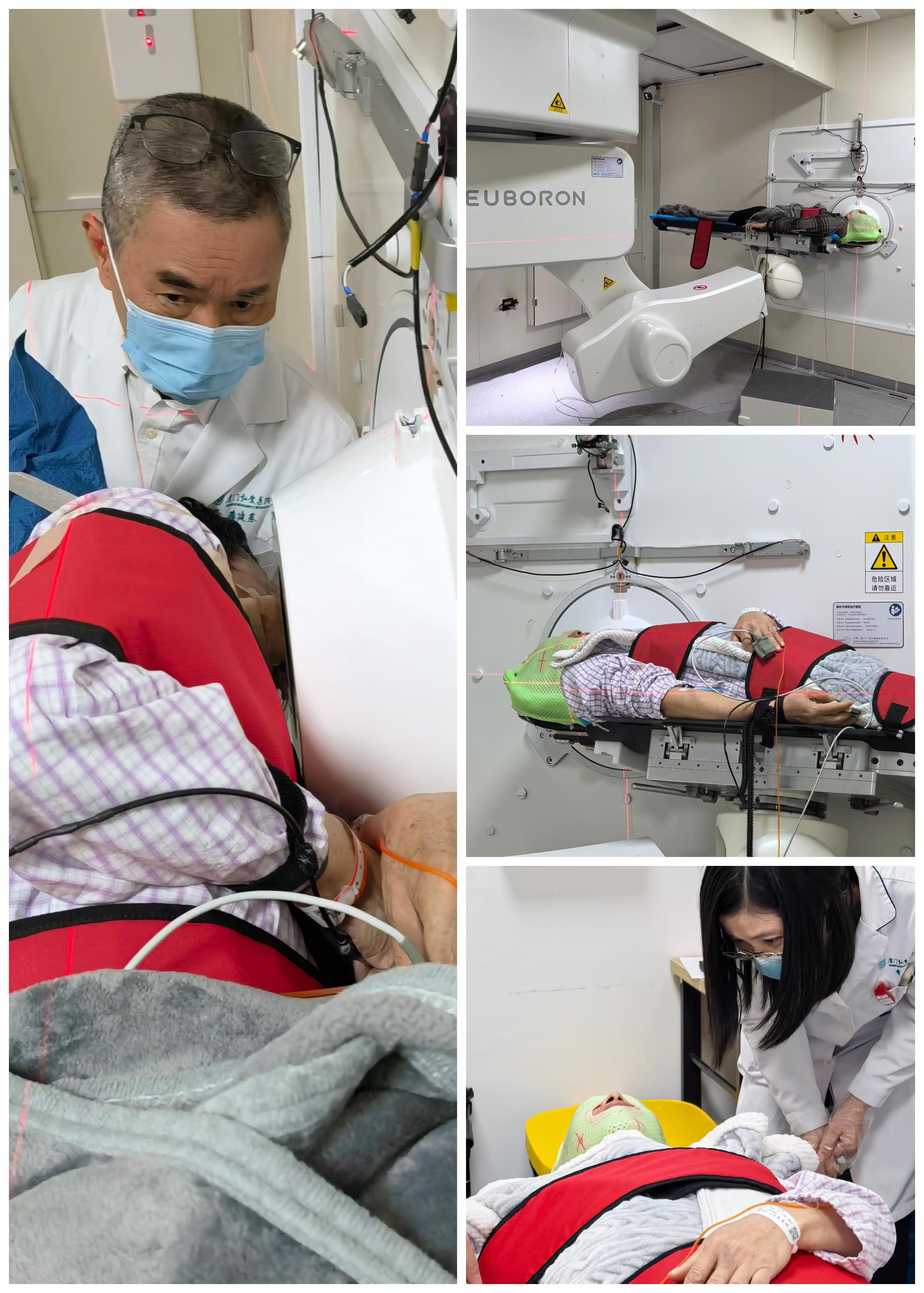
As early as April 2024, Neuboron, relying on its self-developed BNCT system (NeuPEX) and the first boron-targeted therapeutic drug (BPA), launched a BNCT drug-device combined Phase I registered clinical trial at Xiamen Humanity Hospital. Led by Principal Investigators (PIs) Professor Pan Jianji and Professor Huang Cheng, the clinical trial consists of two key phases: Phase Ia (dose escalation) and Phase Ib (dose expansion). At present, Xiamen Humanity Hospital has successfully completed the Phase Ia clinical trial. The enrolled patients generally showed good safety and tolerability, with preliminary good clinical efficacy observed. The Phase Ib clinical trial has also been officially initiated, and the treatment of the first three patients was completed on January 20, with all patients in good condition.
This time, the BNCT companion diagnostic drug containing fluorine [¹⁸F] isotope independently developed by Neuboron is a Class 1 chemical innovative drug. Its indication is: used for positron emission tomography (PET) to locate, diagnose and evaluate solid tumors by utilizing the overexpression of LAT1 in tumors.
In 2022, Neuboron successfully developed a high-yield synthesis route for ¹⁸F-BPA. The production output of a single synthesis can support commercial distribution. It also collaborated with strategic partners such as DC AMS PHARMA and MITRO Biotec (subsidiaries of Dongcheng Pharmaceutical) to conduct preclinical research. In addition, Neuboron collaborated with Director Huo Li from Peking Union Medical College Hospital and Director Zhu Xiaohua from Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology to complete a large number of Investigator-Initiated Trials (IITs), obtaining early safety study data and accumulating preliminary research data for the application of NBB-002.
The use of companion diagnostic drugs can further leverage the key guiding role of imaging, playing a significant role in patient screening for BPA-BNCT, accuracy of dose calculation, follow-up tracking, etc. Meanwhile, preliminary studies at the BNCT Center of Xiamen Humanity Hospital have shown that patients who pass the early screening have a higher possibility of clinical benefit.
The successful clinical approval of Neuboron's domestic innovative BNCT radioactive diagnostic drug and the official initiation of the domestic BPA Phase Ib registered clinical trial mark that China has continuously achieved major milestone breakthroughs in the clinical translation and application of BNCT. In the future, Neuboron will collaborate with Xiamen Humanity Hospital to further advance the market launch and clinical application process of BNCT products, bringing this advanced technology to more patients as soon as possible.